Amasya ’da Gezilecek Yerler ve Amasya Gezi Notları
Amasya ’da Gezilecek Yerler hakkında bilgi vermeye başlamadan önce Amasya gezisi ve Şehzadeler Şehri Amasya hakkında kısa bir bilgi vererek yazımıza başlayalım.
Amasya’nın içinden ya da kenarından otobüsle ve araçla çok geçmeme rağmen (belki de 100’ün üzerinde) bir türlü gezememiştim, Amasya’yı. Genelde bir yerlere gidiyor oluyorduk, gece sabaha karşı ya da akşam üzeri geçiyorduk. 30 Ekim 2016’da otobüsle tekrar geçmem gerekti. Otobüs biletimi sabahın erken saatinde Amasya’da olacak şekilde ayarladım. Gezgin gözüyle gezecek şekilde dersime çalıştım, notlarımı yanıma aldım ve yola çıktım. Planımı da akşama kadar şehri gezdikten sonra akşam üzeri tekrar yola devam edecek şekilde ayarladım.
Sabah ezanından önce Amasya’ya ulaştım. Sokaklar bomboş ve ıpıssızdı. Hafifte bir yağmur yağıyordu. Tek tük köpeklere rastladım, beni görünce kuyruk sallıyorlardı. Kuyruk salladıklarına göre tecrübeme istinaden sorun yoktu. Ben gönül rahatlığı ile sokaklarda ve Yeşil Irmağın kenarında dolaşabilirdim. Öyle de yaptım. Derken tüm camilerden sabah ezanı okunmaya başladı. Minarelerden çıkan ezan sesleri ve seslerin kayalıklardan yansıması değişik bir ortam oluşturuyordu. Sessizliğin ve sabahın dinginliğinin üzerine müthiş bir ses ve sonra tekrar sessizlik. Bu ortam sanırım ben de biraz duygusallık da yapmıştı.

Şehzadeler Şehri Amasya ‘dan sabah saatlerinden bir görüntü
Sonra tan yeri ağarmaya, tan yeri ağardıkça ışıklar sönmeye, başladı. Yeşil Irmağın dinginliğini izleyerek, seher vakti kahvaltı yapacak bir yer buldum. İyi bir kahvaltıdan sonra planladığım ve dersime çalıştığım şekilde Amasya’yı gezmeye başladım.
Şehzadeler şehri olarak da isimlendirilmiş olan Amasya, Orta Karadeniz Bölgesinde yer almaktadır. Yeşil Irmağın ikiye böldüğü kent, kayalar arasına kurulmuş bir nehir kentidir. Nehrin kale tarafında eski şehir, diğer tarafta ise yeni şehir yer almaktadır. Amasya Kalesi’nin de bulunduğu eski şehir bölgesinde; tarihi evler, konaklar ve kaya mezarlıkları gibi birçok tarihi yapı yer almaktadır.
Yapılan arkeolojik kazılara göre Amasya’nın kuruluşu, M.Ö. 2000’li yıllara kadar dayandığı söyleniyor. Hititler, Frigler, Medler ve Perslere ev sahipliği yapan Amasya, ilerleyen yıllarda Romalılar, Bizanslılar, Selçuklular ve Osmanlılara da ev sahipliği yapmış. Fatih Sultan Mehmet dahil birçok Osmanlı padişahın şehzadelik döneminde vali olarak görev yaptığı sancak şehri Amasya, bu nedenle Şehzadeler Kenti olarak anılmaktadır. Bu kapsamda Amasya ’da Gezilecek Yerler ve görülmeye değer birçok tarihi yapı ve tarihi eser var.
Amasya ’da Gezilecek Yerler
Arkeoloji Müzesi, 2’inci Beyazıt Külliyesi, Minyatür Amasya Müzesi, Şehzadeler Gezi Yolu, Alçak Köprü, Şehzadeler Müzesi, Hazeranlar Konağı, Kral Kaya Mezarları, Yalıboyu Evleri, Amasya Saat Kulesi, Sabuncuoğlu Tıp ve Tarih Müzesi, Tarım Müzesi, Millî Mücadele Müzesi ve Amasya Kalesi Amasya ’da Gezilecek Yerler Listesindeki en önemli yerler. Söz konusu yerlerin tamamı Yeşil Irmak boyunca uzandığından ötürü düzenli bir şekilde gezebilmek için Arkeoloji Müzesi’nden Amasya ’da Gezilecek Yerler ve görülmeye değer yerleri gezmeye başlanması en uygun yöntem.
1. Arkeoloji Müzesi
Mustafa Kemal Paşa Caddesi güney tarafında yer alan Arkeoloji Müzesi, Kalkotik Çağlardan (M.Ö. 4000’li yıllar) başlayan 12 ayrı medeniyete ait tarihi eserlere ev sahipliği yapıyor. Amasya’nın çok eskiye dayanan bir tarihi geçmişi olması münasebetiyle söz konusu eserler oldukça fazla. Müzede, başta Hitit ve Roma dönemi olmak üzere birçok medeniyetin izlerini sürmek mümkün. Müzenin ikinci katında bulunan özel bir salonda ise 14.yüzyıla ait 7 adet mumya var. Bahse konu mumyalar, o dönemdeki şehir yöneticiliğini yapmış kişilere ve bu kişilerin ailelerine ait. Müzenin bahçesinde ise oldukça sağlam gözüken lahitler yer alıyor.

Şehzadeler Şehri Amasya Arkeoloji Müzesi
Arkeoloji Müzesi’nden doğuya doğru Mustafa Kemal Paşa Caddesi üzerinde 200 m kadar yürüdükten sonra sol tarafta 2’inci Beyazıt Külliyesi var.
2. İkinci Beyazıt Külliyesi
Yeşil Irmağın güney kıyılarında uzanan Ziya Paşa Bulvarı ile Mustafa Kemal Paşa Caddesi arasında yer alan 2’inci Beyazıt Külliyesi, 1481-1485 yılları arasında Amasya Valisi Şehzade Ahmet tarafından babası 2’inci Beyazıt adına yaptırılmış. Söz konusu külliye; cami, medrese, imaret ve şadırvan şeklinde inşa edilmiş.

Amasya ’da Gezilecek Yerler; 2’inci Beyazıt Külliyesi
Yan mekanlı cami mimarisi şeklinde yapılmış cami bölümü; taç kapısı, sarkıtları ve kitabesi ile ön plana çıkıyor. Günümüzde medrese olarak yapılan bölüm kütüphane, imaret olarak yapılan bölüm ise Maket Amasya Müzesi olarak kullanılıyor.
3. Minyatür Maket Amasya Müzesi
İkinci Beyazıt Külliyesi’nin imarethane olarak inşa edilen bölümde hizmet veren Minyatür Amasya Maket Müzesi, yaklaşık 300 metrekare genişliğinde bir salon. Amasya’nın 100 yıl kadar önceki halini 1/150 ölçeğinde birebir küçülterek yapılmış. Söz konusu maketin yapımında 1914 yılında çekilmiş bir fotoğraftan yararlanılmış. Sadece mimari yapıları ve mekanları göstermek yerine Amasya’nın yaşam biçimini de yansıtacak şekilde tasarlanmış.

Amasya ’da Gezilecek Yerler; Minyatür Amasya Müzesi
Bu bölgedeki geziden sonra Yeşil Irmak ile Ziya Paşa Bulvarı arasında kalan Şehzadeler Yolundan doğuya doğru 400 m yürüyüp Alçak Köprü’den karşı kıyıya geçince hemen sağda Şehzadeler Müzesi var.
4. Şehzadeler Yolu
Şehzadeler yolu, günümüzde kent sakinlerinin nehir kenarında yürüyüş yaptığı, gezdiği yolun bir bölümü. Yol kenarına arkaları nehre bakan Amasya’da valilik yapmış şehzadelerin büstleri yer alıyor. Her büstte ise ilgili şehzadenin kimlik bilgileri bulunuyor.

Amasya ’da Gezilecek Yerler; Şehzadeler Yolu
5. Alçak Köprü
Amasya Kalesi ve güney eteklerinde oluşan eski şehir, zamanında kent merkeziymiş. Romalılar döneminde Yeşil Irmağın karşı kıyısını kent merkezine bağlamak için bu köprü yapılmış. Söz konusu köprü, düzgün kesme taşlarla yapılmış ve dört ayak üzerine inşa edilmiş. Ancak Yeşil Irmak yatağının zamanla yükselmesi sonucu bu dört ayağın sadece üst kısımları görünür kalmış. Köprü uzantısı da bu durumda su yüzeyine yaklaştığından zamanla yöre halkı tarafından Alçak Köprü diye adlandırılmaya başlanmış ve bugünkü ismini almış. 19.yüzyıla kadar bu haliyle hizmet veren köprü, zamanın yöneticileri tarafından bu haliyle tehlike arz edeceği düşünülerek aynı ayakların üzerine 11 ayaklı yeni bir ahşap köprü kurmuşlar. Ancak bu ahşap köprü de Yeşil Irmağın suları arasında kaybolup gitmiş. Ardından kullanılmayan bir kilisenin taşlarından, yeniden bir köprü daha inşa edilmiş. 1965 yılında da bu köprü biraz daha güçlendirilmiş. 1965 yılında güçlendirilen bu söz konusu köprü günümüze kadar gelmiş. Köprüyü inceledikten sonra karşı kıyıdaki Şehzadeler Müzesi ziyaret edilebilir.
6. Şehzadeler Müzesi
Alçak Köprü’nün eski şehir bölümündeki ayağında ve Yeşil Irmağın kıyısındaki eski surların üzerine yapılmış olan iki katlı ahşap bina, Şehzadeler Müzesi olarak restore edilmiş ve bu yeni konumuna uygun olarak yeniden tasarlanmış. Tavan ve duvarları özgün hat ve desenlerle süslenmiş. Ahşap tabanı ise dokunması, renkleri ve motifleriyle geleneksel halı dokumacılığının en güzel örneklerini veren halılarla kapatılmış. Söz konusu bina, adeta 15 ve 16.yüzyıl Anadolu Türk evinin örneği gibi duruyor.

15 ve 16.yüzyıl Anadolu Evi Örneği
Şehzadeliklerini Amasya’da geçirmiş olan Osmanlı sultanlarının aslına uygun resimlerinden yola çıkılarak yaptırılan heykeller ve şehzadelerin kendi dönemlerindeki kıyafetler, müzede öne çıkan hususlar. Müzenin üst katında padişah olmuş şehzadeler, alt katta ise padişah olamamış şehzadeler yer alıyor.

Amasya ’da Gezilecek Yerler; Şehzadeler Müzesi
Buradan sonra Şehzadeler Müzesinin hemen güneybatı bitişiğinde yer alan Hazeranlar Konağı gezisi ile Yalıboyu evlerindeki gezimize devam edebiliriz.
7. Hazeranlar Konağı
1800’lü yılların geleneksel mimarisinin en güzel örneklerinden birisi olan Hazeranlar Konağı, 1872 yılında zamanın defterdarı Hasan Talat Efendi’nin kız kardeşi Hazeran Hanım tarafından yaptırılmış. İki giriş kapısı olan konağın, on bir odası restore edilerek müze haline getirilmiş. Konağın bir kapısı hemen yanı başında bulunan Hatuniye Camine doğru açılıyor. Caminin diğer yanında ise Yıldız Hamamı yer alıyor. Hazeranlar Konağının bulunduğu bölge, Yalıboyu evleri diye de adlandırılıyor ve söz konusu konağa benzer birçok evler var. Bu evler için, Amasya ’da Gezilecek Yerler Listesinin en göze hitap edeni denilebilir.
8. Yalıboyu Evleri
Yalıboyu evleri, arkası Amasya Kalesine ve Kral Kaya Mezarlarına dönük; önden ise Yeşil Irmağa bakan ve nehrin kuzey uzanımı hattına doğru sıralanmış, birbirine benzeyen birçok evden oluşuyor. Amasya evleri olarak da bilinen söz konusu evler, cumbalı ve kafesli Osmanlı mimarisinin en güzel örneği olduğu söyleniyor.

Amasya/Yalıboyu Evleri
Haremlik selamlık bölümlerden oluşan evler, bodrum üzeri tek katlı ya da iki katlı olarak yapılmış. Bu evlerin hemen kuzey bitişiğine bulunan Kral Kaya Mezarları gezisi ile Amasya gezimize devam edebiliriz.
9. Kral Kaya Mezarları
Yalıboyu evleri ile Amasya Kalesi arasında, Harşena Dağı’nın güney eteklerinde bulunan Kral Kaya Mezarları, Pontus Kralı tarafından anıt mezar olarak yapıldığı söyleniyor. Kalker kayalıkların oyularak yapıldığı söz konusu mezarlardan, Yeşil Irmak Vadisi’nde irili ufaklı 23 civarında kaya mezarı varmış. Mezarlar ile Kale arasında kalan Kızlar Manastırı, Osmanlı şehzadeleri tarafından harem olarak kullanılmış.

Amasya ’da Gezilecek Yerler; Kral Kaya Mezarları
Kaya Mezarlarından tekrar aşağıya Yalıboyu evlerine inip batıya doğru Yalıboyu evlerini gezerek ara sokaklardan yürüdüğümüzde eski şehri, yeni şehir merkezine bağlayan köprünün başında Saat Kulesi var.
10. Saat Kulesi
Valilik Binası ile köprü arasında, köprünün kuzey tarafında bulunan saat kulesi, ilk kez 1865 yılında Amasya Valisi Ziya Paşa tarafından yapılmış. Yeni köprünün 1940 yılında inşası esnasın söz konusu kule hasar gördüğü için yıkılmış. Ancak 2002 yılında gerçeğine uygun bir şekilde yeniden yapılmış.

Amasya ’da Gezilecek Yerler; Saat Kulesi
Amasyalılar açısında Saat Kulesi’nin ayrı bir anlamının olduğuna dair kuşaktan kuşağa anlatılan birçok rivayetler var. Saat Kulesini gezip köprüden karşıya geçildiğinde şehir merkezine giriliyor.
11. Şehir Merkezi
Birbirini takip eden, Mustafa Kemal Paşa Caddesi ile Atatürk Caddelerinin ve aynı zamanda Nehir boyunca birbirini takip eden Ziya Paşa Bulvarı ile Mehmet Paşa Caddesi burada, Atatürk Anıtı’nın olduğu şehir merkezinde birleşiyor. Bu meydanda 14.yüyılda kare plana göre kesme taştan yapılmış, ahşap kubbeli Gümüşlü Cami de yer alıyor. Gümüşlü Cami’den batı istikametine doğru 250 metre kadar yüründüğünde Sabuncuoğlu Tıp ve Tarih Müzesine ulaşılıyor.
12. Sabuncuoğlu Tıp ve Tarih Müzesi
Anadolu’nun ilk akıl hastanesi ve ilk şifa merkezi olan Sabuncuoğlu Tıp ve Tarih Müzesi, yöre halkı arasında Bimarhane olarak da biliniyor. Selçuklu Prensesi Yıldız Hatun tarafından 1308 yılında yaptırılan şifahane, Fatih Sultan Mehmet zamanında en parlak dönemlerini yaşamış ve 18.yüzyıla kadar işlevini sürdürmüş. 1385 yılında Amasya’da doğmuş olan ve müzeye de ismini veren Şerafettin Sabuncuoğlu, söz konusu şifahanede çalışmış hekimlerin en ünlüsüymüş. Şerafettin Sabuncuoğlu, yazdığı eserleri ve eserlerinin bilimsel değerleriyle tıp tarihçileri tarafından önemli bilginler arasına yerleştirilmiş.
Sabuncuoğlu Tıp ve Tarih Müzesi’nde; Sabuncuoğlu’nun uzmanlık dalları ve kullandığı aletler ile yaptığı çalışmaların anlatıldığı resimler ve maketler, müzikle tedavi çalışmaları ve söz konusu çalışmaların canlandırılması ile zengin bir İslami müzik aletleri koleksiyonu sergileniyor.

Amasya ’da Gezilecek Yerler; Büyükağa Medresesi
Sabuncuoğlu Tıp ve Tarih Müzesi’nden doğu istikametine doğru tarihi Kunç Köprüsü’ne kadar, nehir kenarından Mehmet Paşa caddesini takip ederek, 400 metre civarında yüründüğünde, sırasıyla; Mustafa Bey Hamamı, Mehmet Paşa Cami, Kumacuk Hamamı, Kunç Köprüsü, Kunç Köprüsü’nden nehrin karşı kıyısına geçince solda yapay bir şelale, sağda ise sırasıyla Büyükağa Medresesi ve Millî Mücadele Müzesi de gezip görülecek yerler arasında.
13. Millî Mücadele Müzesi
Resmi tam adı, Saraydüzü Kışla Binası Millî Mücadele Müzesi ve Kongre Merkezi olan söz konusu bina, orijinaline uygun olarak sonradan Yeşil Irmak kıyısına yeniden inşa edilmiş. Binanın tarihi önemi ise Mustafa Kemal Atatürk’ün 1919 yılı Haziran Ayında Amasya’ya gelişinde üs olarak kullandığı ve Amasya Tamiminin kaleme alındığı yer olması. Söz konusu bina, Cumhuriyet Dönemine ait eserlerin ve bazı belgelerin sergilendiği bir yer olmasının yanında aynı zamanda bir kültür merkezi olarak da kullanılıyor.

Amasya ’da Gezilecek Yerler; Milli Mücadele Müzesi
Milli mücadele Müzesi için, Amasya ’da Gezilecek Yerler Listesinin en anlamlısı denebilir. Mustafa Kemal’in Amasya’ya gelişi, karşılama heyeti ve Amasya Tamiminin yayımlanışını anlatan rölyefler ile heykeller, bu döneme ait bazı belgeler, Millî Mücadele Müzesi’nde sergilenen en önemli eserler diyebiliriz. Buradaki gezinin arkasından Amasya Kalesine çıkarak, Amasya’yı birazda tepeden seyredip Amasya gezisi sonuçlandırılabilir.
14. Amasya Kalesi
Amasya Kalesi, Millî Mücadele Müzesi’ne; yürüyerek 2700 metre, araç ile 3200 metre mesafede yer alıyor. Dolambaçlı Kale yolunu takip ederek sürekli yokuş yukarı çıkılıyor. Amasya, iki büyük dağ silsilesinin arasından geçen bir vadinin içine kurulmuş. Amasya Kalesi ise bu söz konusu iki dağ silsilesinin kuzeyinde bulunan Harşena Dağı’nın zirvesine yapılmış. Bu nedenle Amasya Kalesi’ne Harşena Kalesi de deniyor.

Amasya ’da Gezilecek Yerler; Amasya Kalesi
Deniz seviyesinden 700, Amasya şehir merkezinden 300 metre yükseklikte bulunan kale; bir rivayete göre Pontus Kralı Mithridates tarafından, başka bir rivayete göre ise Kumandan Harsana tarafından yapıldığı söylenmektedir. Harşena Kalesi diye de adlandırılmasının sebebi buna bağlanmaktadır. Amasya Kalesi için Amasya ’da Gezilecek Yerler Listesinin en eski ve tarihi olanı diyebiliriz.
Tarihi kale birçok kez el değiştirmiş, sırasıyla; Perslerin, Romalıların, Bizanslıların, Selçukluların ve Osmanlıların hakimiyetine girmiş. Her saldırıda büyük zararlar gören kale, her seferinde onarılmış ve yeniden yaptırılmış. Özellikle 1075 yılında Türklerin eline geçtikten sonra önemli bir onarım görmüş ve 18.yüzyıla kadar kullanılmış, sonra da askeri önemini kaybetmiş.
Kalenin tepesi, kesme taşlar ve sur duvarlar ile moloz taşlardan yapılmış. Kale, İçeri Şehir (hatuniye mahallesi, Amasya/Yalı evlerinin bulunduğu bölge), Kızlar Sarayı (kaderine terk edilmiş durumda) ve Yukarı Kale (en tepe nokta) olmak üzere üç bölümden oluşuyor. Amasya gezisini, Yukarı Kale’de bitirmek şehrin fotoğrafının akılda kalması için en iyi yöntem.

Amasya Kalesi’nin En Tepe Noktası, Yorgun ve Perişan Halim
İstanbul’dan 11 saatlik bir gece yolculuğuna müteakip Amasya’ya gelmek, sabahın karanlığında geziye başlamak, akşama kadar yürüyerek Amasya ‘da Gezilecek Yerler ve görülecek yerleri bir plan dahilinde gezmek, sonra da akşamleyin tekrar otobüsle yolculuğa devam etmek; gezme virüsünün insana bulaşması böyle bir şey…!!!
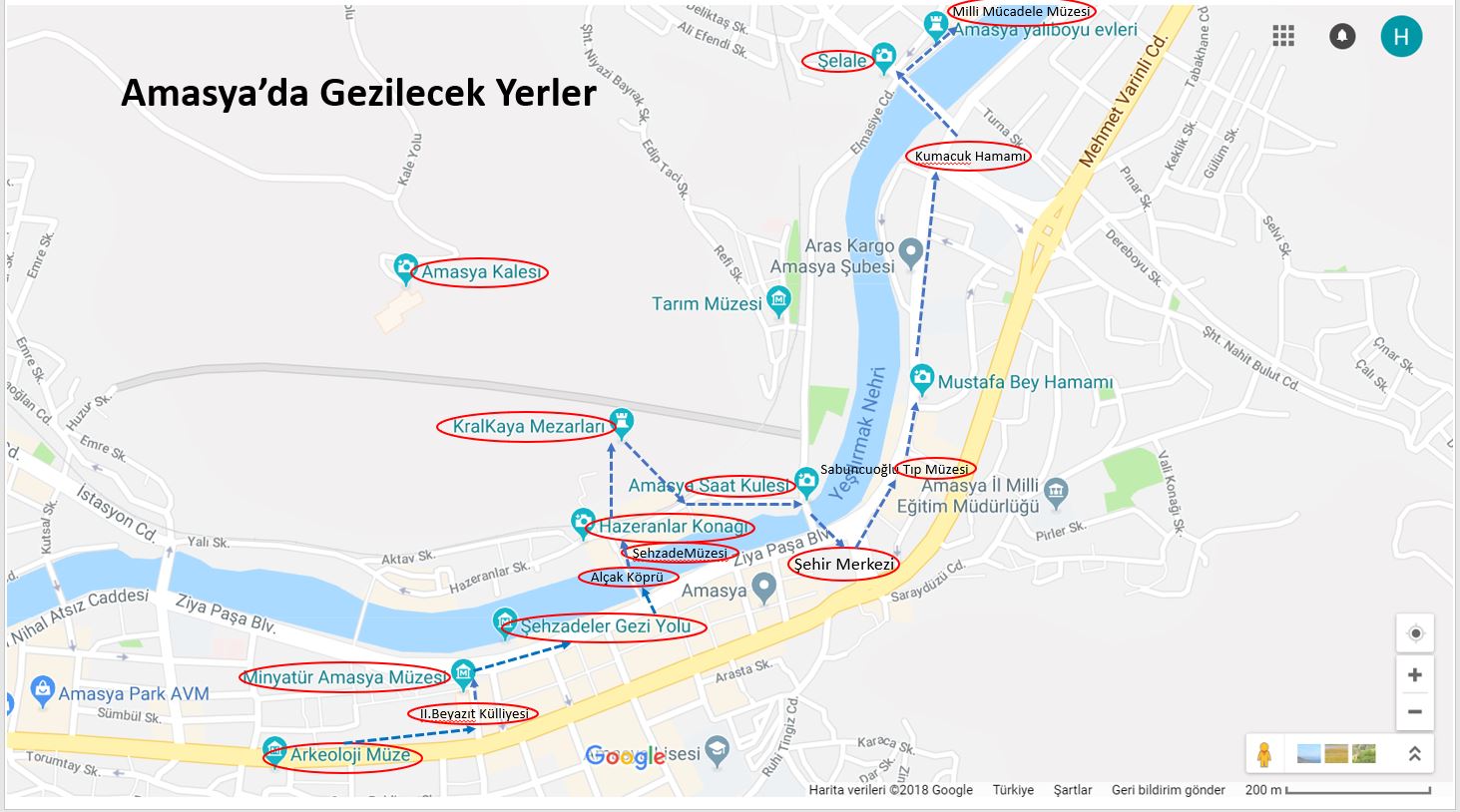
Amasya ‘da Gezilecek Yerler
***
Amasya ’da Gezilecek Yerler birbirine oldukça yakın ve yürüyüş mesafesinde. Tüm bu yerler yürüyerek bir günde gezilebilir. Amasya’ya 120 km mesafede Karedeniz sahilinde bulunan Samsun ve Samsun’a 150 km, Amasya’ya ise 250 km mesafede yer alan sahil kenti Sinop, aynı gezi programı içerisinde gezilebilir. Bu kapsamda aşağıda verdiğimiz linkleri tıklayarak ilgili yerler hakkında bilgi sahibi olunabilir.

