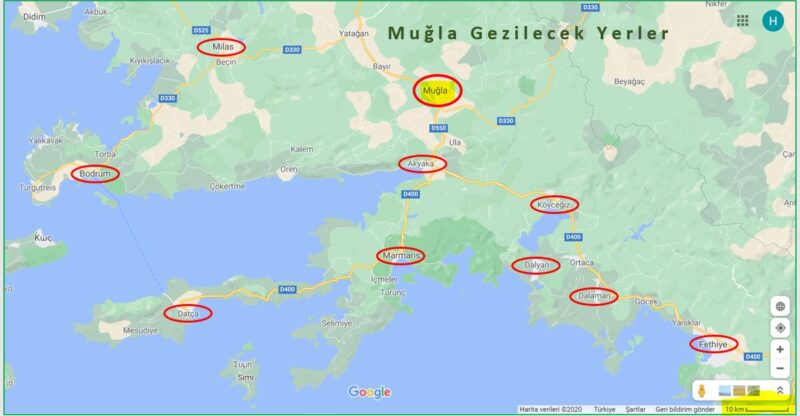
Muğla Gezilecek Yerler, Muğla Hakkında Bilgi
Muğla gezilecek yerler bakımından oldukça zengin bir çeşitliliğe sahip popüler bir turizm şehrimiz. Bu popülerliğini özellikle birbirinden meşhur turizm ve tatil kentleri olan Marmaris gibi, Bodrum ve Fethiye gibi ilçelerinden alıyor.
Geçmişi M.Ö 3400’lü yıllara kadar dayanan Muğla ve civarı özellikle Karya ve Likya medeniyetleri ile ön plana çıkıyor. Muğla gezilecek yerler ve söz konusu yerler hakkında bilgi vermeye başlamadan önce; Muğla nerede, Muğla’ya nasıl gidilir sorularını kısaca cevaplayalım.
Muğla nerede
Güneybatı Anadolu bölgesinde yer alan Muğla, Batı Akdeniz sahilinde çok önemli bir turizm şehrimizdir. Muğla araçla; İzmir’e 210 km, İstanbul’a 670 km, Ankara’ya 620 km, Antalya’ya 310 km, Dalaman Havaalanı’na 95 km, Milas-Bodrum Havaalanına 77 km mesafede yer almaktadır.
Muğla’ya nasıl gidilir
Uçakla, Dalaman Havaalanı’na ya da Milas-Bodrum havaalanına gelip, havaalanından toplu taşıma ya da araç kiralayarak Muğla’nın her tarafına gidilebilir. Söz konusu havaalanlarına, Pegasus ve benzeri özel uçak şirketleri ile THY ’nın ve THY’nın yan kuruluşu olan Anadolu Jet uçak firmalarının seferleri mevcut.
Anadolu’nun birçok kentinden kalkan şehirler arası otobüs ile de Muğla’nın her ilçesine her tatil beldesine gidilebilir. Uçak biletlerini turna.com ve benzeri ucuzabilet.com gibi internet sitelerinden, otobüs biletlerini ise obilet.com gibi internet sitelerinden fiyat ve zaman kıyaslaması yapılarak en uygun biletler alınabilir.
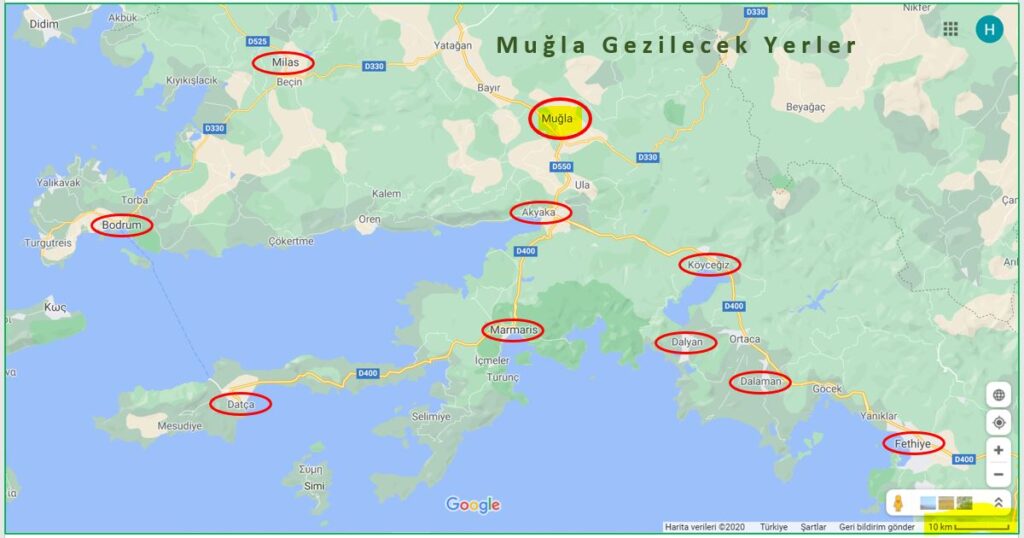
Muğla Gezilecek Yerler
Muğla nerede, Muğla’ya nasıl gidilir sorularının kısa yanıtından sonra Muğla gezilecek yerler ve Muğla gezilecek yerler hakkında bilgi vermeye başlayabiliriz.
Muğla Gezilecek Yerler
Doğası, denizi, koyları, gölleri ve tarihi eserleri ile ön plana çıkan Muğla ve ilçeleri, tarihte birçok medeniyete ev sahipliği yapmış. Dünyaca bilinen Karya Yolu ve Likya Yolu gibi tarihi kültür yollar da Muğla’da yer alıyor. Bu bölge Bodrum, Datça, Marmaris, Köyceğiz, Ortaca, Dalyan ve Fethiye gibi tatil yerleriyle Dünya’nın her ülkesinden turist çekme potansiyeline sahip.
Marmaris
Mavi yolculuk turlarının öncüsü Marmaris; içmelere kadar uzanan 16 km’lik muhteşem kumsalları ve plajları, oldukça şık restoranları, kafeleri ve barları, tarih kokan Kaleiçi ve çevresi, liman ve çevresi, tarihi yapıları, gölü andıran denizi, kenti çepeçevre sarmış olan çam ormanları ile mutlaka görülmesi gereken bir tatil beldesi.

Marmaris Gezilecek Yerler, Liman ve Kale
Yılın ortalama 300 günü sıcak yüzünü esirgemeyen pırıl pırıl güneşi ile de ön plana çıkan Marmaris, renkli ve eğlenceli gece hayatı ile de ön plana çıkıyor. Kent merkezinin yanında, merkezden oldukça kolay ulaşılabilen köyleri ve masmavi koyları, adaları, yarım adalarıyla da Marmaris herkes için adeta bir çekim merkezi.
Özellikle Bozburun Yarımadası’nın doğu sahilinde ve batı sahilinde birbirine oldukça yakın olan İçmeler, Turunç, Hisarönü, Orhaniye, Turgutlu, Bayır, Selimiye, Bozburun ve Söğüt ile Gökova Körfezi’nin doğu ucunda yer alan Akyarlar Marmaris’in çevresinde dikkat çeken ve görülmesi gereken önemli tatil beldeleri. Marmaris gezilecek yerler ve gezilecek yerler hakkında detaylı bilgiler için tıklayınız…
Dalyan
Caretta deniz kaplumbağalarıyla anılan Dalyan; sessiz ve sakinliğiyle, gölleriyle, sazlıklarıyla, sanki bir labirent gibi iç içe geçmiş su kanallarıyla dikkat çekiyor. Dalyan, aynı zaman Karyalıların MÖ 6. yüzyılda kurduğu Kaunos şehrinin kalıntılarına da ev sahipliği yapıyor.

Muğla Gezilecek Yerler, Dalyan
Yeşili andıran rengiyle Dalyan Boğazı, Dalyan merkezin hemen karşısındaki kayalıklarda yer alan Kral Mezarları, Boğazın Akdeniz’le birleştiği yerden başlayan ve caretta carettaların yumurtlama alanı olan meşhur İztuzu Plajı, Gökbel ve Gökbel Dağı, Köyceğiz Gölü tarafında yer alan Sultaniye Kaplıcaları, Köyceğiz Gölü ve Köyceğiz Dalyan bölgesinde gezip görülmesi gereken başlıca yerler. Dalyan gezilecek yerler ve gezilecek yerler hakkında detaylı bilgiler için tıklayınız…
Datça
Yaz ve kış mevsimleri arsındaki ısı farkının az olduğu Datça, özellikle yaz aylarında kuzeyden esen meltemin sıcağı ve nemi yok etmesiyle ön plana çıkıyor. Bölgeye has bu hava durumu, tüm Datça ve çevresinde hüküm sürüyor. Bölgenin bu özelliği nedeniyle Datça ve çevresi ile tüm yarımada birçok kimsenin tatil ve dinlenme için tercih ettiği bir yer durumundadır.

Muğla Gezilecek Yerler, Datça
Datça Merkez, Datça Limanı, Kumluk Plajı, Taşlık Plajı, Eski Datça, Kargı Koyu Datça Merkezde gezilecek başlıca yerler. Marmaris yolu üzerinde Karaincir Plajı, Datça Yarımadası’nın en uç noktasında Knidos Antik Kenti de gezilecek yerler arasında.
Datça yarımadasının güney güney batı ucundaki sahilde yer alan Palamutbükü, Akvaryum Plajı, Ovabükü, Hayıtbükü, Datça’nın çevresinde gezip görülecek başlıca diğer yerler. Datça gezilecek yerler ve gezilecek yerler hakkında detaylı bilgiler için tıklayınız…
Fethiye
Ülkemizin en önde gelen turizm ve tatil merkezlerinden birisi olan Fethiye; özellikle doğal güzellikleri ve tarihsel zenginlikleriyle ön plana çıkıyor. Birçok antik kenti, doğası, denizi ve değişik doğa aktiviteleri ile oldukça fazla çeşitlilik gösteren Fethiye, birçok tatil ve turistik merkezlere yakınlığıyla da dikkat çekiyor.

Fethiye Ölüdeniz
Daha çok Ölüdeniz, Kelebekler Vadisi, Kayaköy, Saklıkent, Kabak Koyu gibi merkezden uzaktaki yerlerle anılan Fethiye, şehir merkezinde de vakit geçirip gezilebilecek birçok yere sahip. Söz konusu yerlerden Fethiye Müzesi, Amintas Kaya Mezarları, Liman ve çevresi, Çalışlar Plajı ve sahili bunlardan en çok ön plana çıkanlar. Fethiye gezilecek yerler ve gezilecek yerler hakkında detaylı bilgiler için tıklayınız…
Akyaka
Türkiye’nin en harika kumsallarının birinin kıyısında yer alan Akyaka; çam ormanlarıyla kaplı dağları, akvaryum kadar berrak pırıl suları, kraliçelere layık kumsalları, eski Muğla mimarisini yaşatan evleriyle ön plana çıkıyor.
Gökova Körfezi ile adeta bütünleşmiş olan Akyaka, “Uluslararası Yavaş Şehir Birliği” üyesi şirin bir turistik ve tatil beldesidir. Kendine has bir mimari yapısı olan bu belde, birçok tatil ve turistik merkezlere yakınlığıyla da dikkat çekiyor.

Muğla Gezilecek Yerler, Akyaka
Muğla’dan Marmaris ve Fethiye’ye giderken, Ula’yı geçip dolambaçlı yollardan aşağı doğru inmeye başlayınca birdenbire Gökova Körfezi karşımıza çıkıyor. Körfezin en doğu ucunda Sakartepe’nin eteğinde yer alan Akyaka’yı gezmeye, kendine has mimari yapısıyla ön plana çıkan Akyaka evlerini gezip görerek başlanabilir.
Akyaka merkezde ve Akyaka çevresinde; Akyaka evleri, Azmak Çayı ve Kadın Azmağı, Orman Kampı, Akçapınar Plajı, Kleopatra Plajı, Sedir Adası başlıca gezip görülecek yerler. Akyaka gezilecek yerler ve gezilecek yerler hakkında detaylı bilgiler için tıklayınız…
Köyceğiz
Köyceğiz Gölü ile adeta özdeşmiş durumda olan Köyceğiz; sessiz ve sakinliğiyle, narenciye bahçeleriyle, kuzeyindeki çam ormanlarıyla kaplı dağlarla ön plana çıkıyor. Sessizlik ve sakinliğini sanki gölden almış gibi duran bu göl kasabasının merkezinde ve çevresinde gezip görülebilecek birçok yer var.

Köyceğiz Gölü
Köyceğiz Merkez, Köyceğiz Gölü, Dalyan, Sultaniye kaplıcaları, Ekincik Koyu, Yayla Köyü, Gökçeova Gölü, Yuvarlak Çay Yeşil Vadi bölgesi Köyceğiz’de gezip görülecek başlıca yerler. Akyaka gezilecek yerler ve gezilecek yerler hakkında detaylı bilgiler için tıklayınız…
Bodrum
Meşhur beyaz kireç kaplı ve çivit mavisi kapı pencereli taş evleriyle öne çıkan Bodrum, Türkiye’nin en popüler tatil yerlerinin başında yer alıyor. Yılın neredeyse altı ayı yaz sıcaklarının yaşandığı bu turizm kenti, sadece Türk halkı için değil yabancı turistler içinde en çok tercih edilen yerlerin başında yer alıyor.

Bodrum
Uğruna şiirler yazılan, şarkılar bestelenen, hikayeler romanlar yazılan Bodrum; gezip görülecek birçok değişik yere sahip. Bodrum Kalesi, Tavşan Adası, Sualtı Arkeoloji Müzesi, Pedasa Antik Şehri, Myndos Kapısı, Zeki Müren Müzesi, Antik Tiyatro, Halikarnas Mozolesi, değişik özelliklerde irili ufaklı birçok Bodrum Koyu Bodrum’da gezilecek başlıca yerler arasında. Bodrum gezilecek yerler ve gezilecek yerler hakkında detaylı bilgiler için tıklayınız…
Milas
Antik kentleri, plajları, tarihi eserleri, doğa güzellikleriyle adeta bir cennetten farkı olmayan Milas; Bodrum gibi, Marmaris gibi popüler yerlerin biraz gölgesinde kalmış bir turizm kentimiz. Milas, söz konusu popüler tatil yerlerin hareketli ve hızlı yaşamının yanında sakinliğini, sessizliğini ve huzurlu ortamını korumuş denebilir. Bu kapsamda bakıldığında Milas’ta gezip görülecek birçok yer var.

Milas Gümüşkesen Anıt Mezarı
Firuz Bey Cami, Milas Müzesi, Hacıaliağa Konağı, Çöllüoğlu Hanı, Gümüşkesen Mezar Anıtı, Beçin Kalesi, Iassos Antik Kenti, Herakleia Antik Kenti, Zeytinlikuyu Plajı, Bafa Gölü Milas’ta gezip görülecek başlıca yerler. Milas gezilecek yerler ve gezilecek yerler hakkında detaylı bilgiler için tıklayınız…