Ürdün Gezi Programı ve Gezi Rotası
Ürdün Gezi Programı ve gezi rotası; değişik gezi kitapları ve gezi blogları incelenerek hazırlandı. Ayrıca ayrıntılı harita çalışması yapılmış, google map’teki gezgin yorumlarından da faydalanılmıştır. Bu kaynaklardan edinilen bilgiler kapsamında öncelikle Ürdün Gezilecek Yerler ‘in listesi çıkarılarak bilgi notu hazırlandı. Ardından Ürdün Gezi Programı oluşturuldu.
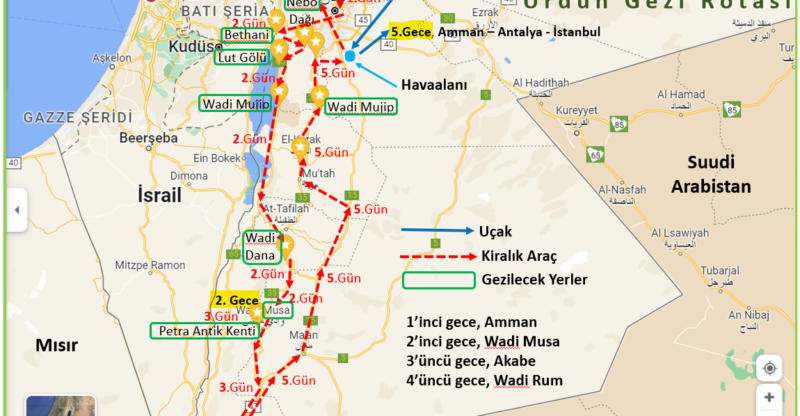
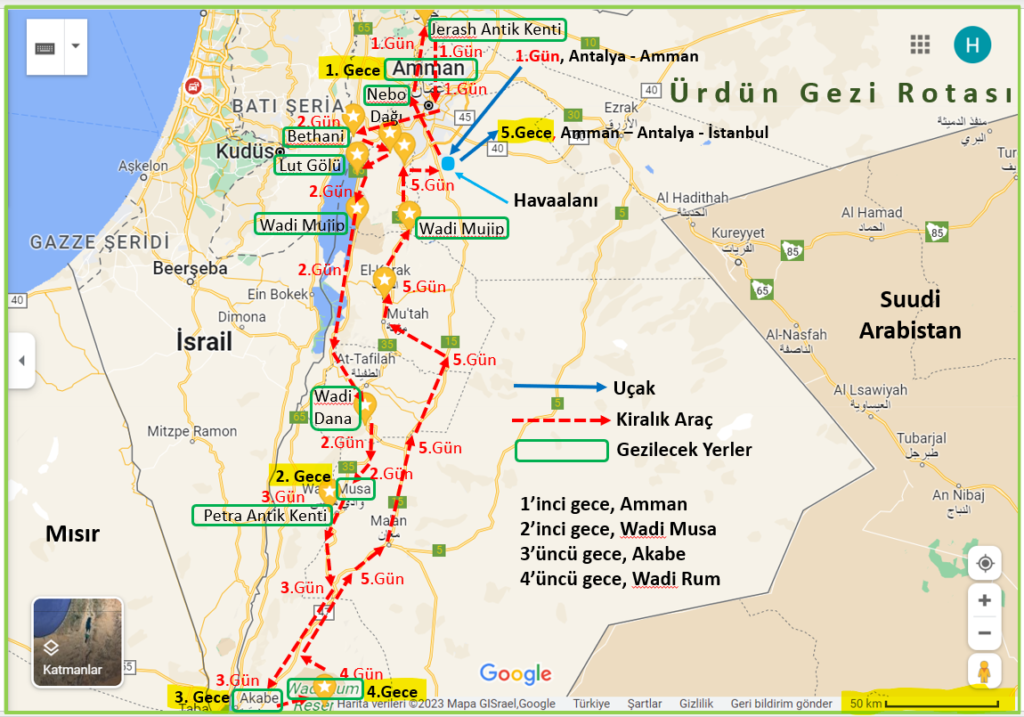
Araba kiralayarak gezmenin daha kolay olacağı bu ülkede, tüm gezilecek yerler bir güzergahta toplanmış durumda. Bu güzergah, ülkenin kuzeyinde yer alan başkent Amman ile güneyinde yer alan Akabe arasında.
Bu iki kent arasındaki mesafe araçla 330 km civarında. Amman, Jerash Antik Kenti, Bethani, Lut Gölü (Dead Sea), Nebo Dağı, Medeba, Wadi Mujip, Kerak Kalesi, Wadi Dana, Petra Antik Kenti, Akabe, Hicaz Demiryolu Tarihi Treni, Wadi Rum, Ürdün’de gezip görülmeye değer başlıca yerler.
Bizim Ürdün Gezi Programı
06 Mart 2023 Pazartesi günü, saat 21.30’da Antalya Uluslararası Havalimanı’nda buluşuyoruz. Ardından pasaport ve benzeri diğer işlemlerimizi saat 24.00’e kadar tamamlıyoruz.
1.Gün, 07 Mart 2023 Salı; ANTALYA – AMMAN – JERASH ANTİK KENTİ – AMMAN
Pasaport ve benzeri diğer işlemlerden sonra saat 00.30 gibi uçağımıza biniyoruz. Ardından saat: 01.00’de Ürdün’ün başkenti’ne Pegasus Hava Yolları ile hareket ediyoruz. Bir buçuk saatlik yolculuğun ardından saat 02.30 gibi Ürdün’ün turistik şehirlerinden birisi olan Amman’a ulaşıyoruz. Ardından pasaport kontrolü ve benzeri işlemlerimizi tamamlıyoruz.
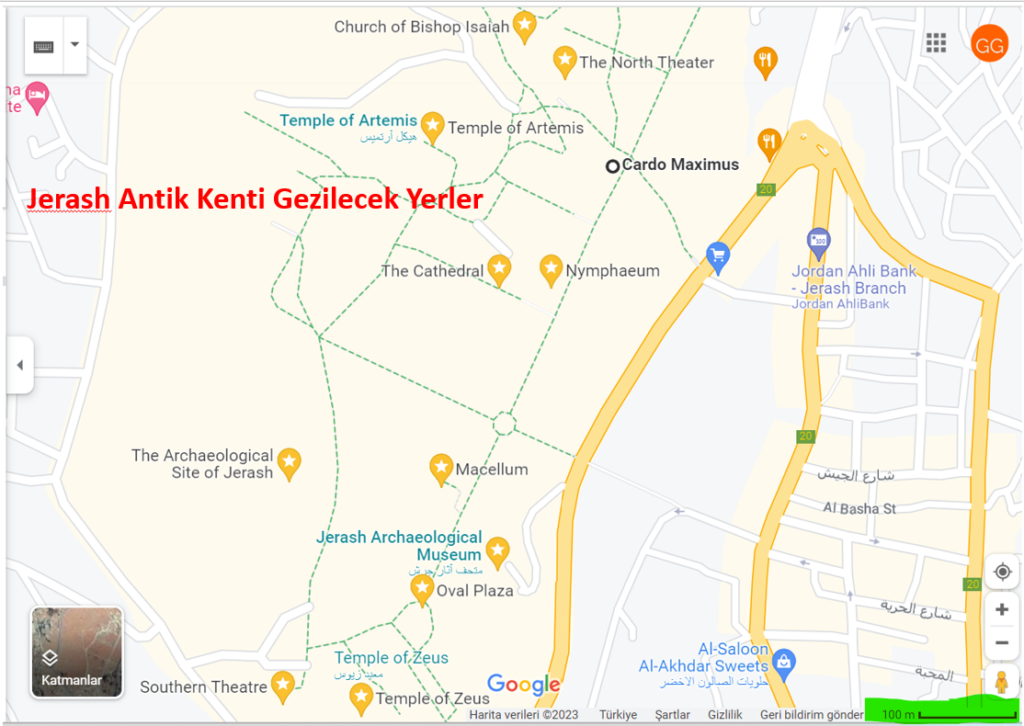
Tüm gezi boyunca bizim her türlü (çöl hariç) ulaşımımızı sağlayacak olan aracımızı kiralıyoruz. Ardından gün aydınlanınca aracımızla Amman’ın 51 km kuzeyinde yer alan Jerash Antik Kenti’ne hareket ediyoruz.
Son yıllarda yıldızı parlayan bu Antik Kent’in, Ürdün’de Petra’dan sonra en çok ziyaret edilen tarihi yerlerden birisi olduğu söyleniyor. Uygun bir yerdeki kahvaltıdan sonra bu antik kenti gezip görüyoruz.
***
Ortadoğu’da en iyi durumda bulunan bu Roma şehirlerini gezip gördükten sonra Amman’a dönüyoruz. Ve bir gece konaklayacağımız otelimize yerleşiyoruz. Ardından yürüyerek şehir gezimize başlıyoruz.
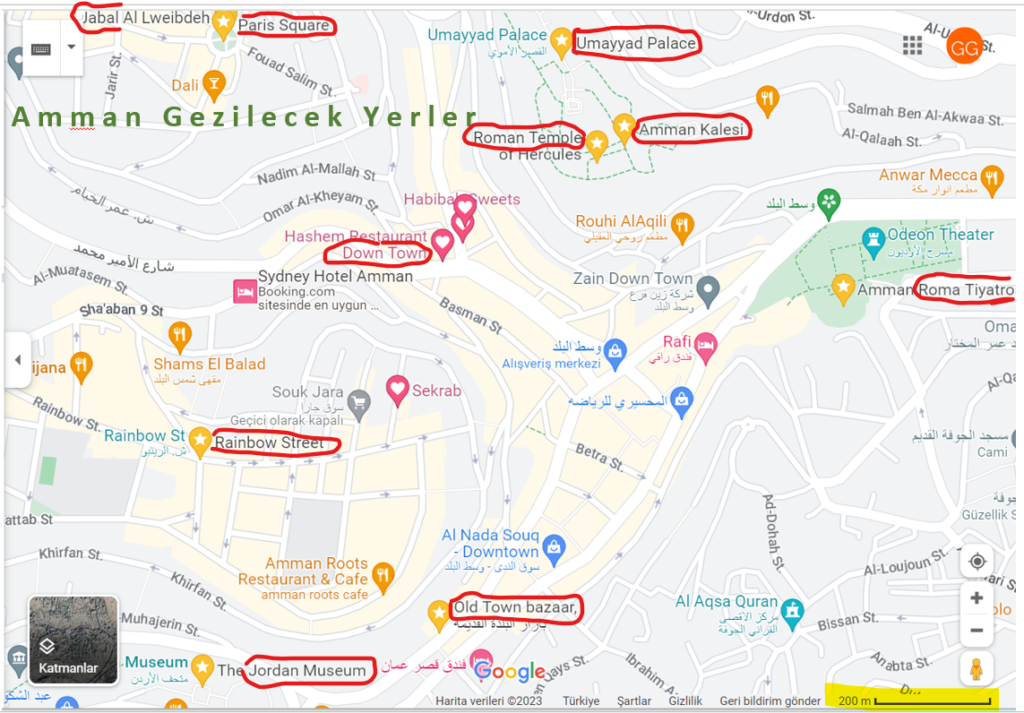
Bu kentin en önemli tarihi eseri, Romalılardan kalma Amman Kalesidir. Kale bölgesinde Romalılardan kalma antik bir tiyatro, Arap döneminden kalma nispeten korunabilmiş bir saray, bir de Roma döneminden kalma bir tapınak bulunuyor. Tapınağın bulunduğu yer bize iyi bir şehir manzarası sunuyor.
Hediyelik eşya ve gümüş takıların ağırlıklı olarak satıldığı eski tarihi Pazar, Ürdün’ün tarihi gelişimini anlatan Ürdün Arkeoloji Müzesi, Amman’ın karmaşasından uzakta ve değişik yemek çeşitleri ile kafelerin olduğu Rainbow Street, Modern alışveriş merkezlerinin yer aldığı Abdali Bulvarı, Kaleye karşı konuşlanmış ve birçok sokağın çıktığı etrafında kafelerin olduğu Paris Meydanı Amman’da gezip görmeye değer diğer yerler.
Keyifli bir akşam yemeğinin ardında Ürdün Gezi Programı ‘nın ilk gecelemesi, Amman’da POST boutique hotel ‘de…
2.Gün, 08 Mart 2023 Çarşamba; AMMAN – LUT GÖLÜ – WADİ MUSA
Otelimizde yapacağımız sabah kahvaltısına müteakip erkenden yola koyuluyoruz. Bugün 400 – 450 km’lik bir yol yaparak Petra Antik Kentine ev sahipliği yapan bir gece konaklayacağımız Wadi Musa kentine gideceğiz. Rotamız, Amman > Bethany (El Mağtas) > Nebo Dağı > Lut Gölü > Wadi Mujib > Wadi Dana > Wadi Musa (Petra Antik Kenti) olacak.
Bugünkü rotamız üzerinde birçok gezip görülmeye değer yerler var. Zamanımız elverdiği müddetçe hepsini de gezip görmeye çalışacağız. Zamanımız el vermez ise bazılarını iptal edebiliriz.
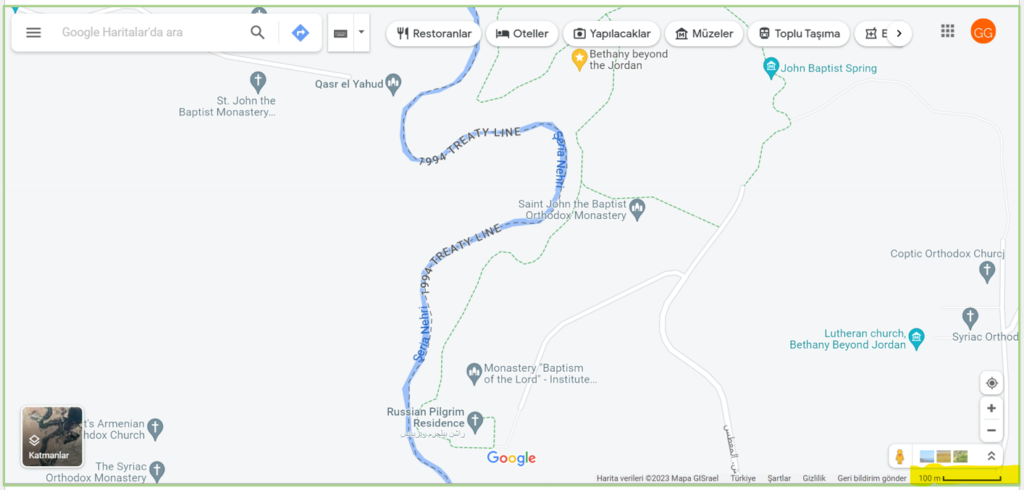
Bugün sabah saatlerinde ilk göreceğimiz yer, Bethany (El Mağtas) olacak. Bu kutsal yer, Amman’ın araçla 52 km güney batısında yer alıyor. Vaftiz Siti adı ile de bilinen bu arkeolojik bölge, aynı zamanda UNESCO Dünya Mirası alanı.
***
Bethany’den sonra göreceğimiz ikinci nokta ise yüksekliği ile bölgeye tamamen hâkim olan Nebo Dağı. Bu dağ, Bethany (El Mağtas)’nin araçla 30 km güney doğusunda yer alıyor. Bölgeden Jeriko, Ramallah, Kudüs ve Lut gölü gibi birçok yer ve şehrin göründüğü söyleniyor. Ayrıca bu dağın, Hz. Musa’nın Mısır dan kaçarken İsrailoğulları’na vadedilmiş toprakları gösterdiği ilk yer olduğu da söylenenler arasında.
Bugün üçüncü göreceğimiz yer, dünyada başka bir örneği olmayan Lut Gölü. Eksi 430 metrelik rakımıyla dünyanın deniz seviyesinden en düşük rakımına sahip, bu göl. Gölün kenarında aracımızla 80 km civarında bir yolculuğumuz da olacak. Bu yolda gölün oluşturduğu değişik doğa oluşumlarını da göreceğiz.
Son olarak göreceğimiz yer Ürdün’ün en büyük doğa rezervi olduğu söylenen Dana Biyosfer Alanı. Burada Dana Köyü ve Wadi Dana’yı gördükten sonra bir gece konaklayacağımız Wadi Musa’ya hareket ediyoruz. Ürdün Gezi Programı ‘nin ikinci gecelemesi Wadi Dana’daki otelimizde.
3.Gün, 09 Mart 2023 Perşembe; WADİ MUSA – PETRA – WADİ MUSA – AKABE
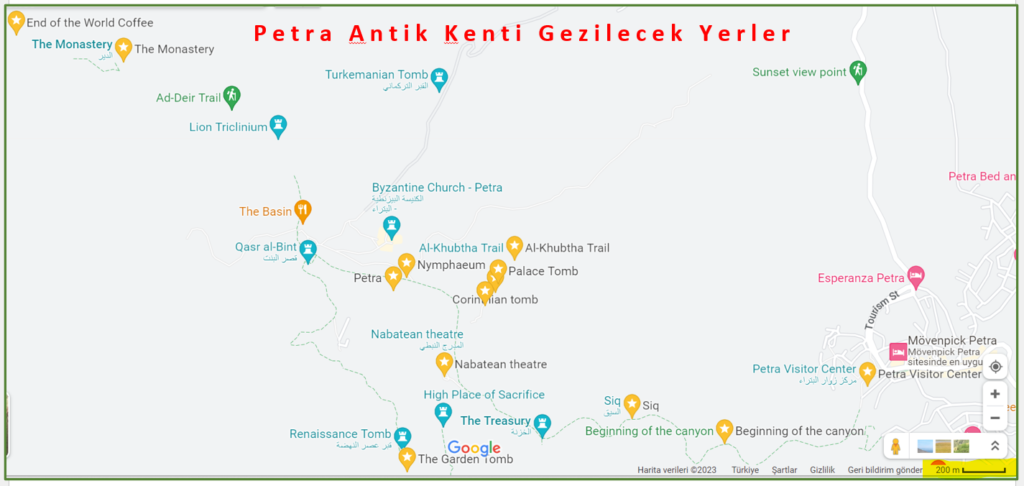
Otelimizde yapacağımız kahvaltının ardından bugün tam gün Ürdün ile simgeleşmiş olan Petra Antik Kenti’ndeyiz. Buradaki tarihi yapıların çoğunluğu, yüksek kayalarla çevrili 6-7 km’lik bir ana yol güzergahında. Bir kısmı da bu yola çıkan ara yollar üzerinde bulunuyor.
Gezimizin ana konusu olan bu Antik Kentte, kentin dikkat çeken en görkemli yapılarından Al Khazneh (Hazine), Roma tarzında inşa edilmiş Amfitiyatro, Ad – Deir Manastırı, tapınaklar, kaya mezarlarının bulunduğu geniş kanyon Street of Facades, Kraliyet Mezarları, Hz Musa’nın Kardeşi Harun’un Mezarı (Aaron’un Mezarı).
Petra Antik Kenti’ndeki gezinin ardından Petra’nın araçla 125 km güneyinde yer alan Akabe şehrine hareket ediyoruz. Akabe Körfezi’nin kıyısında yer alan Ürdün’ün bu küçük Liman Kenti’ne varışa müteakip otelimize yerleşiyoruz. Ardından şehirde kısa bir keşif turuna yapıyoruz. Müteakiben keyifli bir akşam yemeğinin ardından Ürdün Gezi Programı ‘nın üçüncü gecelemesi Akabe’de Dweik Hotel 2 ‘de…
4.Gün, 10 Mart 2023 Cuma; AKABE – HİCAZ DEMİR YOLLARI – WADİ RUM
Yılın 300 günü güneşli olan Akabe’de otelimizde yapacağımız kahvaltının ardından gezimizin diğer bir ana konusu olan Wadi Rum’a doğru yola koyuluyoruz. Akabe’nin 70 km kuzey doğusunda yer alan Wadi Rum’a doğru giderken ilk önce yolumuzun 45’inci km’sinde bulunan Hicaz Demir Yolları’nı gezip görüyoruz. Ardından öğlene doğru Wadi Rum’a ulaşıyor ve bir gece konaklayacağımız kampımıza yerleşiyoruz.
2011 yılında UNESCO Dünya Kültür Mirası Listesi’ne alınan Wadi Rum’da birçok arkeolojik alan ve kaya oluşumları ile yeryüzü oluşumları var. Burada herhangi bir alan seçilip, 4-5 saat süren bir jeep safari turu yapılabilir. Günbatımının kızıllığı, gecenin sessizliği, yıldızların parıltısı yaşandıktan sonra Ürdün Gezi Programı ‘nın dördüncü ve son geceleme noktası Wadi Rum’da otelimizde.
5.Gün, 11 Mart 2023 Cumartesi; WADİ RUM – KERAK – MADABA – AMMAN HAVAALANI
Wadi Rum’da sabahın erken saatlerinde gün doğumu kızıllığını yaşadıktan sonra kampımızda sabah kahvaltımızı yapıyoruz. Ardından dönüş yoluna koyuluyoruz. Gezimizin ikinci günündeki gibi uzun bir yolumuz ve yolumuz üzerinde gezip göreceğimiz birkaç yer var.
Dönüş yolunda ilk göreceğimiz yer, Wadi Rum’un 240 km kuzeyinde yer alan Kerak Kalesi. Bu kale, El-Kerak kentinde bulunuyor. Eski kral Yolu boyunca seyahat eden birçok kişinin diğer turistik yerlere giderken uğradığı bu Haçlı Kalesi, 1140’lı yıllarda Kudüs Latin Krallığındaki haçlı devletlerini korumak için inşa edilmiş.
***
Bugün göreceğimiz diğer bir yer ise Kerak Kalesi’nin 40 km kuzeyinde yer alan Wadi Mujip. Armon Çayı’nın oluşturduğu vadi, deniz seviyesinden 420 m aşağıda yer alıyor. 2011 yılında da UNESCO Biyosfer Alanı ilan edilerek koruma altına alınmış olan bu vadide, değişik zorluklarda ve değişik kategorilerde suyu takip eden doğa yürüyüşü ve benzeri doğa sporları yapılabilir.
Dönüş yolunda göreceğimiz son nokta, Madaba mozaik haritası. Kutsal Toprakların bilinen en eski haritası olan bu harita, Wadi Mujib’in araçla 50 km kuzeyinde yer alan Medeba şehrinde bir kilisenin zemininde bulunuyor. Bu haritayı gezip gördükten sonra Ürdün’ün beşinci büyük kentinde son akşam yemeğimizi yiyor ve ardından Amman Havaalanına geçiyoruz.
Önce kiralık aracımızı havaalanında teslim ediyoruz. Ardından pasaport ve çıkış işlemlerini yapıyoruz. Sonra saat: 03.40’taki uçağımızın kalkış saatini bekliyoruz.
6.Gün, 12 Mart 2023 Pazar; AMMAN – ANTALYA – İSTANBUL
Pasaport işlemlerimize müteakip Amman’dan gece saat 03.40’ta Pegasus Havayolları ile İstanbul’a hareket ediyoruz. Bir buçuk saatlik bir uçak yolculuğunun ardından saat 05.15’te Antalya’dayız.
Antalya Havaalanında giriş işlemlerimizi yaptıktan sonra saat: 09.35’te Pegasus Havayolları ile İstanbul’a hareket ediyoruz. Bir saat 20 dk.lık bir uçak yolculuğunun ardından saat: 10.55’te İstanbul’dayız.
***
KONAKLAMA YERLERİ :
Amman, 1 gece / 7 Mart, Salı
Wadi Musa, 1 gece / 8 Mart, Çarşamba
Akabe, 1 gece / 9 Mart, Perşembe
Wadi Rum, 1 gece / 10 Mart, Cuma